Fluid static | Complete notes | Important questions | Short questions and Numerical problem solutions | NEB Physics | Physics in Depth
Fluid is a substance that can flow. Fluids take the shape of a container on which we put them. Fluid flows because they cannot withstand the shearing stress (i.e., tangential force per unit area). However, it can exert a force in the direction perpendiular to its surface. Liquids and gases are fluids. Some characteristics properties of a fluid can be described by its density and pressure.
Density
Density of a substance is defined as the ratio of mass per unit volume. \[\rho=\frac{m}{V}\] Here, \(\rho\) is the density of the substance with mass m and volume V. Density is a scalar quantity and its SI unit is \(kg/m^3\).
Pressure
Pressure is defined as the force per unit area. \[P=\frac{F}{A}\]
Here, F is the magnitude of normal force on area A. Pressure is a scalar quantity and its SI unit is \(N/m^2\) or Pa (Pascal).
Pressure exerted by a liquid at a depth h is, \[P=h\rho g\]
Here, \(\rho\) is the density of liquid and g is the acceleration due to gravity at a given place.
Pascal's law of pressure
It states that when a pressure is applied to an enclosed liquid, the pressure is equally transmitted to every portion of it . When you squeeze one end of a tube to get toothpaste out the other end, you are watching Pascal's principle in action . Pascal's law is applied also in the case of Heimleich maneuver . Here, when a food is stucked in the throat, a sharp pressure is applied in the abdomen propoerly which is transmitted to the throat and the stucked food is ejected.
Upthrust
The upward force exerted by a fluid on an object which is completely or partially immersed in the fluid is called the upthrust or buoyant force.
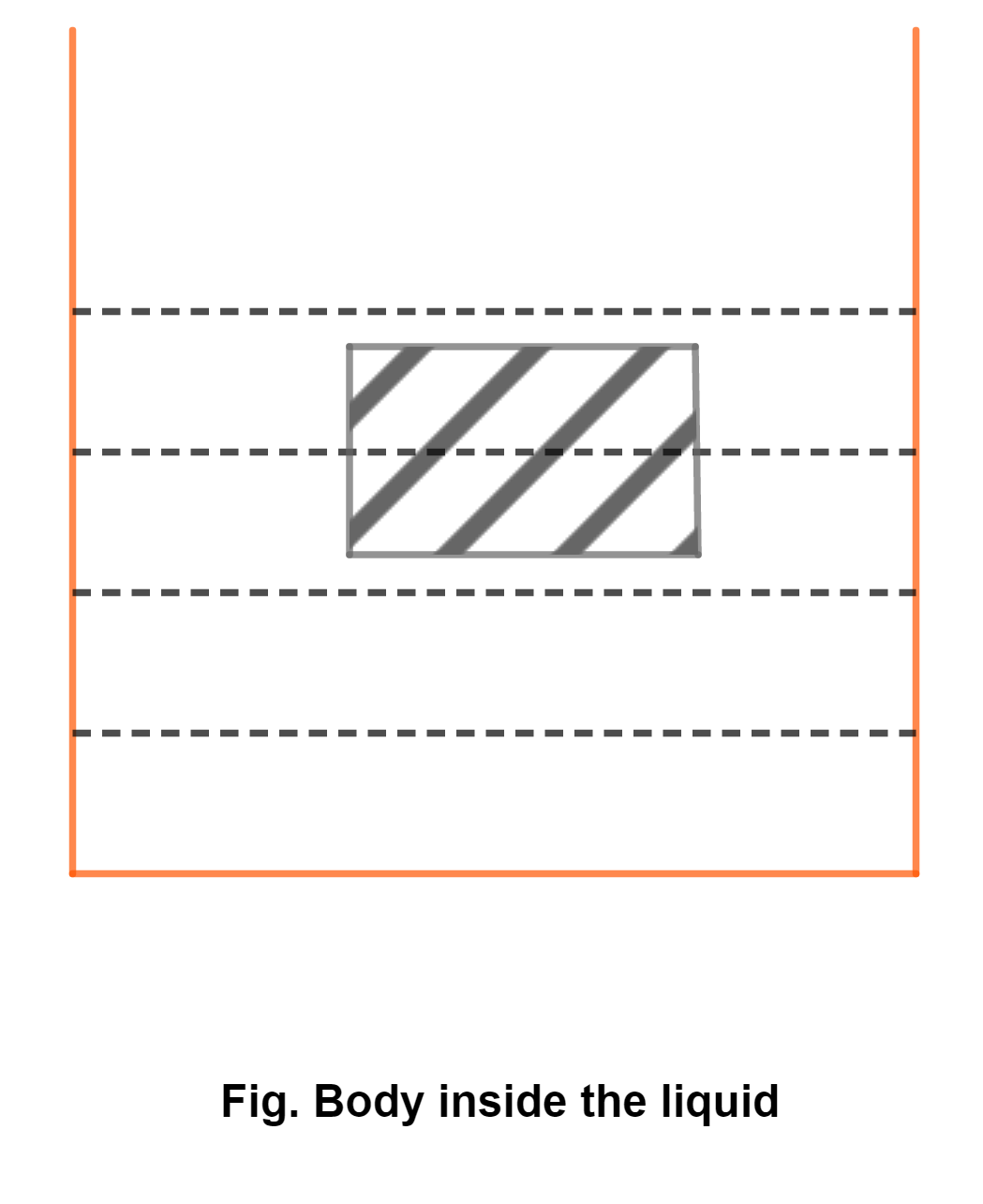
Consider a body immersed in a liquid. When it is completely immersed in a liquid as in fig., pressure at its bottom is greater than at its top. Thus, a net upward force acts on a body due to this pressure difference and thus buoyant force is produced.
Archimede's principle
It states that When a body is fully or partially immersed in a fluid, it experiences an upthrust which is equal to the weight of the fluid displaced by the body. If m be the mass of the displaced fluid of density \(\rho\) and V be the volume of the immersed body, then the upthrust is , \[\text{Upthrust}=\text{weight of the displaced fluid}=mg=\rho V g\]
Floatation
When a body floats on a liquid, the buoyant force or the upthrust on the body is equal to the weight of the body. Since, buoyant force is equal to the weight of the fluid displaced. It can also be said that, "A floating body displaces its own weight of fluid".
ie.,
\[U=m_fg\]
Where, \(m_f\) is the mass of the displaced fluid.
And, \[U=m_bg\]
i.e., for the body to float, upthrust must be equal to the weight of the body of mass \(m_b\).
From this two expressions,
\[\begin{align*}
m_fg&=m_bg\\
\text{weight of the displaced fluid}&=\text{weight of the body}
\end{align*}\]
Consider a block of density \(\rho=800\hspace{0.1cm}kg/m^3\) floats face down in a fluid of density \(\rho_f=1200 \hspace{0.1cm} kg/m^3\)
. The block has height 6 cm. By how much depth the block is submerged?
Here, \(\rho=800\hspace{0.1cm}kg/m^3\) , \(\rho_f=1200 \hspace{0.1cm} kg/m^3\), H = 6 cm = 0.06 m, depth to which the block is submerged h = ?
Let, \(m_b\) be the mass of block with area \(A\) , \(m_f\) be the mass of displaced fluid, \(V_b\) be the volume of block and \(V_f\) be the volume of displaced fluid.
We know that, the body floats when the upthrust on the body matches its own weight i.e., \(U=m_bg\) and also, \(U=m_fg\) .
So, \[\begin{align*}
m_bg&=m_fg\\
\rho V_b&=\rho_f V_f\\
\rho A H&=\rho_f A h \\
h&=\frac{rho}{\rho_f}H\\
&=\frac{800 \times 0.06}{1200}\\
\therefore h&=0.04 \hspace{0.1cm} m
\end{align*}\]
So, the block is submerged by the depth of 0.04 m (or 4 cm).
Specific gravity
Specific gravity of a substance is defined as the ratio of the density of substance to the density of the water at 4\(^\circ\) C. i.e.,
\[\text{Specific gravity}=\frac{\rho}{\rho_w \hspace{0.1cm}\text{at}\hspace{0.1cm} 4^\circ \hspace{0.1cm} C}\]
Cases :
- If specific gravity is less than 1, the object will float on liquid.
- If specific gravity is equal to 1, the object will neither sink nor float i.e., it hovers in the liquid.
- If specific gravity is greater than 1, the object will sink in the liquid.
The density of a copper is 8.96 g/cm\(^3\). Find it's specific gravity.
Here, density of copper, \(\rho= 8960 \hspace{0.1cm} kg/m^3\); density of water at 4 \(^\circ\)C, \(\rho_w=1000 \hspace{0.1cm} kg/m^3\), Specific gracity of copper = ?
\[\begin{align*}
\text{specific gravity}&=\frac{\rho}{\rho_w}\\
&=\frac{8960}{1000}\\
&=8.96
\end{align*}\]
So, the specific gravity of copper is 8.96. Since, the specific gravity of copper is greater than 1, it sinks in water.
A solid weighs 10 g in air and 6.8 g in a liquid whose relative density is 0.8. What is the relative density of the solid?
Here, Weight of the solid in air, \(w_a = 0.01\times 10=0.1\) kg, weight of solid in a liquid, \(w_l\) = 0.0068\times 10=0.068\) kg.
So, the weight of the displaced liquid is , \(w_a-w_l=0.032\) kg
Relative density of the solid with respect to the liquid is,
\[\frac{w_a}{w_a-w_l}=\frac{0.1}{0.032}=3.125\]
Relative density of the solid is,
\[\begin{align*}
&=\frac{\rho}{\rho_w}\\
&=\frac{\rho}{\rho_l}\times \frac{\rho_l}{\rho_w}\\
&=\frac{\rho}{\rho_l} \times \frac{\rho_l}{\rho_w}\\
&=\text{relative density of solid with respect to liquid} \times \text{relative density of liquid}\\
&=3.125\times 0.8 \hspace{0.1cm}\because \text{relative density of liquid}=0.8\\
&=2.5
\end{align*}\]
A geologist finds that a moon rock whose mass is 7.2 kg has an apparent mass 5.88 kg when submerged in water. What is the density of the rock?
Here, Weight of the rock , \(w_1=7.2\times 10=72 \hspace{0.1cm} N\), weight of the rock in water, \(w_2=5.88 \times 10=58.8 \hspace{0.1cm} N\), density of the rock, (\rho\)=?
Relative density of the rock is, \[\begin{align*}
&=\frac{w_1}{w_1-w_2}\\
&=\frac{72}{72-58.8}\\
&=\frac{60}{11}
\end{align*}\]
Also, the relative density of rock is,
\[=\frac{\rho}{\rho_w}\]
So, we can write,
\[\begin{align*}
\frac{60}{11}&=\frac{\rho}{\rho_w}\\
\rho&=\frac{60}{11}\times 1000\\
\therefore \rho&=5454.54 \hspace{0.1cm} kg/m^3
\end{align*}\]
So, the density of the rock is \(5454.54 \hspace{0.1cm} kg/m^3\).
An ice cube floats in water. Will the water level rise if the ice melts completely? Explain.
Let, \(V_i\) be the volume of ice, \(\rho_i\) be it's density ,\(m_w\) be the mass of water displaced by ice, \(V_w\) be the volume of water displaced by the ice (inside water), and \(\rho_w\) be the density of water.
For a floating body,
\[\begin{align*}
\text{weight of ice}&=\text{weight of displaced liquid}\\
m_i g&= m_w g\\
\rho_i V_i&=\rho_w V_w g\\
V_w&=\frac{\rho_i \times V_i}{\rho_w}\\
\end{align*}\]
When ice melts into water,
\[\begin{align*}
\text{resulting volume of water}&=\frac{\text{mass of ice}}{\text{density of water}}\\
&=\frac{\rho_i \times V_i}{\rho_w}\\
\end{align*}\]
Since the volume of water obtained from the melted ice is equal to the volume of ice inside water level, so water level remains the same.
The density of ice is 971 \(kg/m^3\), and the approximate density of sea water in which an iceberg floats is 1025 \(kg/m^3\). What fraction of the iceberg is beneath
the water surface?
Specific gravity of the ice gives the fraction of the iceberg beneath the water surface.
\[\begin{align*}
\text{specific gravity}&=\frac{\rho_i}{\rho_w}\\
&=\frac{971}{1025}\\
&=0.95
\end{align*}\]
So, the fraction of iceberg beneath the water surface is 0.95.
An ice cube of mass 50 g floats on the surface of a strong brine solution of volume 200 cm\(^3\) inside a measuring cylinder. Calculate the level of the liquid in the
measuring cylinder (i) before and (ii) after all the ice is melted. (iii) What happens to the level if the brine is replaced by 200 cm\(^3\) water and 50 g of ice is again added? (Assume density of ice, brine =
900, 1100 kg/m\(^3\) respectively.)
(i) Ice floats on a brine solution if the upthrust on it by the solution equals its weight (i.e., 50 g = 0.05 kg). i.e.,
\[\text{Upthrust}=\rho V g\]
Where, \(\rho\) is the density of the brine solution and V is the volume of the solution displaced.
So, \[V=\frac{0.05}{1100}=4.54\times 10^{-5} \hspace{0.1cm} m^3\]
So, the level on the measuring cylinder after the ice is melted is \(200\times 10^{-6}+4.54\times 10^{-5} \hspace{0.1cm} m^3= 2.454\times 10^{-4} \hspace{0.1cm} m^3\).
(ii) When all the ice is melted, the volume of water is,
\[=\frac{0.05}{\rho_w}=\frac{0.05}{1000}=50\times 10^{-6} \hspace{0.1cm} m^3\]
In this case, the level of the measuring cylinder rises to \(200\times 10^{-6}+50\times 10^{-6} \hspace{0.1cm} m^3= 250\times 10^{-6} \hspace{0.1cm} m^3\).
(iii) In this case, initially the volume of water displaced is \(50 \times 10^{-6}\) because the upthruston ice is equal to its own weight and density of water is 1000 kg/m\(^3\). So, the level on cylinder initially is
\(250\times 10^{-6}\) kg/m\(^3\).
If 1 g of ice melts, volume displaced is 1 \(cm^3\) less. But volume of water formed is 1 \(cm^3\). Thus the net change in water level is zero. Hence the water level remains unchanged as the ice melts.
Click on Hydrostatic questions to find the important questions.
Click on Hydrostatic solutions to find the solutions to short questions and numerical problems.


Comments
Post a Comment